Our Products

SaliCov is an innovative antigen saliva test that detects the presence of COVID-19 in both symptomatic and asymptomatic patients.
This unique tool does not require professional personnel or laboratory equipment, enabling fast and easy screening of large populations.
SaliCov can be performed anywhere by anyone. The broad applicability of this test will enable accurate and rapid diagnostics that will enable schools, workplaces, and cultural attractions to reopen their doors.
Our devices require no external analytic systems, no professional assistance for operating and no electrical power source. The first product that was developed with Salignostics’ breakthrough technologies is the rapid Saliva-Based Pregnancy Home test, which is now under final regulatory procedures for obtaining a CE clearance for EU marketing.
In light of the recent COVID-19 pandemic, Salignostics team took the challenge to adjust the “tool-box” for a rapid, safe, straightforward SARS-CoV-2 diagnostic procedure. This test will share the same technological platform and appearance with the home pregnancy test for a rapid, clear, easy to read “Yes/No” on-the- spot result.

Salignostics LTD is an Israeli biotech start-up company devoted to developing new Saliva-Based-Diagnostic solutions. Our technologies aimed to enable low-cost, rapid and safe diagnostic procedures, wrapped in an esthetic, user friendly design.
Spun-off the Hebrew University of Jerusalem in 2016, Salignostics has a strong scientific background. It was co- founded by 5 experienced Biomedical PhD researchers, leaded by prof. Aaron Palmon, the Dean of the Hebrew University’s Faculty of Dental Medicine. Under Prof. Palmon’s leadership as the company’s president, Salignostics team has developed a series of saliva treatments, comprising a “tool-box” of processing technologies. These technologies enable meeting the full diagnostic potential of saliva by removing its innate hindrances and revealing its lower concentrated analytes free for detection procedures as target molecules.
Our devices require no external analytic systems, no professional assistance for operating and no electrical power source. The first product that was developed with Salignostics’ breakthrough technologies is the rapid Saliva-Based Pregnancy Home test, which is now under final regulatory procedures for obtaining a CE clearance for EU marketing.
In light of the recent COVID-19 pandemic, Salignostics team took the challenge to adjust the “tool-box” for a rapid, safe, straightforward SARS-CoV-2 diagnostic procedure. This test will share the same technological platform and appearance with the home pregnancy test for a rapid, clear, easy to read “Yes/No” on-the- spot result.
Rapid & Easy
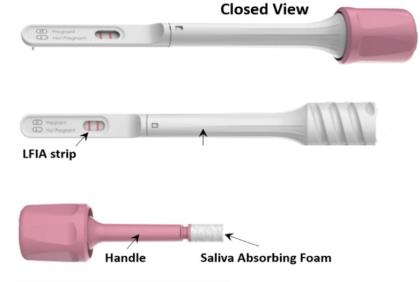
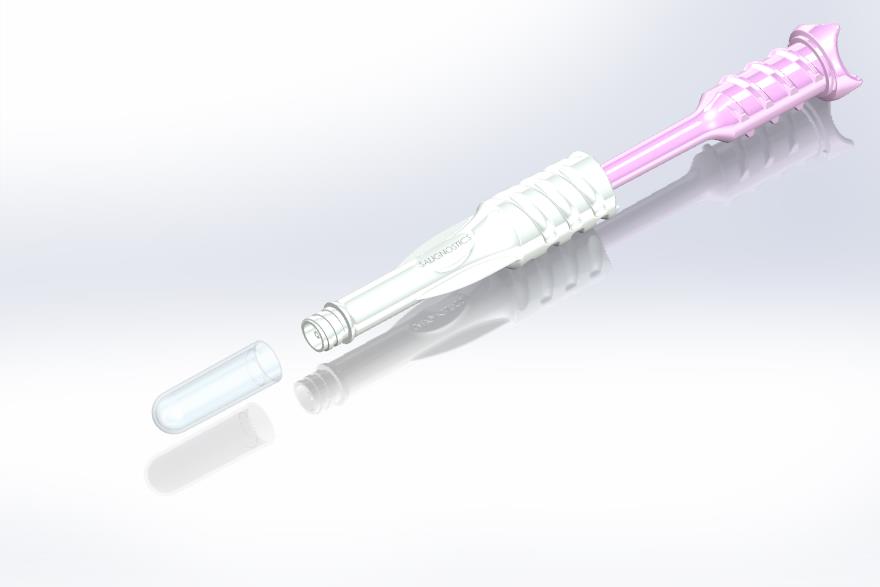
Salignostics’ devices include a collection handle to which a collecting foam is attached. The user independently inserts the foam into the mouth for 60-90 seconds until it absorbs a sufficient amount of saliva, as indicated by the appearance of blue lines along the collecting foam. This collection procedure is simple and allows even young children to collect their own sample. The collecting handle is than inserted into the processing unit. An easy turn-twist mechanism squeezes the saliva into a processing micro- device implemented in the unit. The processed saliva flows to an analytical strip where the result is displayed in the form of a single line (“Healthy”) or two lines (“COVID-19 suspected”). The whole procedure takes only 15 minutes from sample collection to result interpretation.
Better Sample Collection For Better Compliance
Saliva is gaining increased attention as a preferable body-fluid for COVID-19 detection due to the relatively high viral load it has among asymptomatic individuals and early symptomatic patients. Saliva testing enables rapid collection with high compliance from a broader population. Utilizing Salignostics collection method spares the inconvenience related to either nasopharyngeal or oropharyngeal swabs used for most collection procedures today.
Preliminary Results
*Saliva Antigen tests were examined by a digital reader that yields results reflecting the Optical Density (OD) of their test line. OD values of 17.5 or higher were calibrated to the Limit of Detection of the naked eye. Thus: OD>=17.5: Positive result; OD<17.5: Negative result.
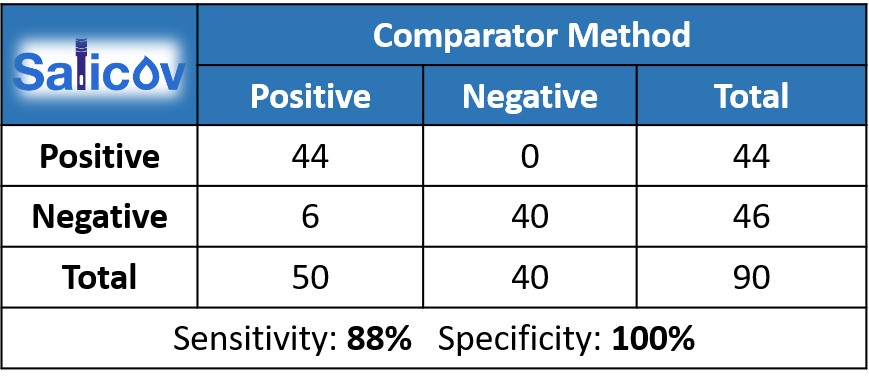
In early SaliCov’s clinical experiments individuals with high to moderate viral load showed Positive results. The same results were obtained with Gold Standard Saliva PCR. Samples with Saliva CT values lower than 29, showed sensitivity of 88% as represented in the Table below. In comparison to nasopharyngeal CT values (<29) the sensitivity level was about 71%. No False Positive results were found (specificity level of 100%).
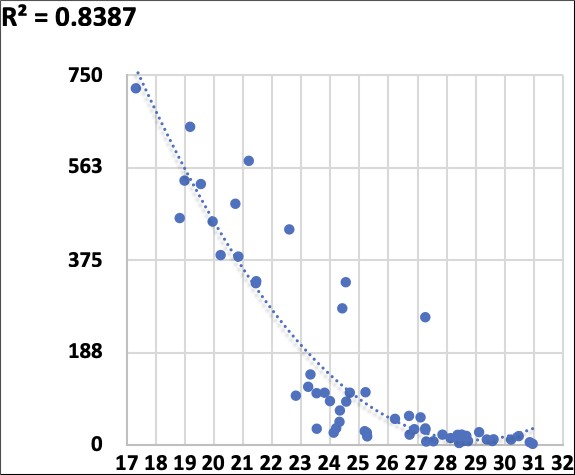
The graph represents the optical density trend of Salignostics’ tests in relation to saliva CT levels. The high negative correlation further depicts the ability of the saliva rapid antigen test to identify high salivary viral load (lower CTs are correlated to higher ODs in Salignostics’ rapid test; R2 = 0.84).
Such viral load indicates high infectivity potential which can now be detected in public places such as schools, theaters, airports, sport arenas, public transportation and more.
Benefits

Results in 15 minutes

Test without a swab

Testing on a community level

Asymptomatic Screening

User-experience
Non-invasive, painless, clean and accessible

Safe
Needle free

Medical Staff
Does not require medical staff, optimal for self and home testing

Rapid
Move quickly from collection to results

“Mirror of the Body”
Reflects a variety of physiological conditions

Accessible
Suitable for monitoring and broad population screening

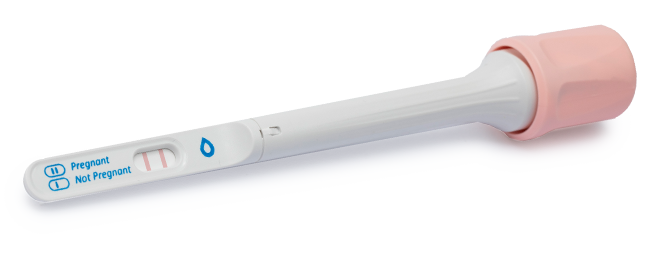
SalistickTM is the first rapid saliva-based pregnancy test with a new and improved user-experience, and high accuracy for early pregnancy detection. The kit is based on our revolutionary technology which detects the pregnancy hormone β-hCG in saliva.
Our technology opens the door for a sensitive detection of the pregnancy hormone β-hCG in saliva. SalistickTM is the first rapid saliva-based pregnancy test for a new and improved user-experience, accompanied by high accuracy for early pregnancy detection.
Our devices do not require external analytic systems, professional assistance for operating and no electrical power source. The first product that was developed which utilizes Salignostics’ breakthrough technologies is the rapid Saliva- Based Pregnancy Home Test, which is currently under final regulatory procedures for obtaining a CE clearance for EU marketing.
In light of the recent COVID-19 pandemic, Salignostics team took the challenge to adjust the “tool-box” for a rapid, safe, straightforward SARS-CoV-2 diagnostic procedure.
BETTER SAMPLE COLLECTION FOR BETTER COMPLIANCE Saliva is gaining increased attention as a preferable body-fluid for COVID-19 detection due to the relatively high viral load it has among a-symptomatic individuals and early symptomatic patients. Saliva testing enables rapid collection with higher compliance from broader population. Utilizing Salignostics collection methods spares the inconvenience related to either Nasopharyngeal or Oropharyngeal swabs used for most collection procedures today.
BETTER SAMPLE COLLECTION FOR BETTER COMPLIANCE Saliva is gaining increased attention as a preferable body-fluid for COVID-19 detection due to the relatively high viral load it has among a-symptomatic individuals and early symptomatic patients. Saliva testing enables rapid collection with higher compliance from broader population. Utilizing Salignostics collection methods spares the inconvenience related to either Nasopharyngeal or Oropharyngeal swabs used for most collection procedures today.
Salignostics’ devices include a collection handle to which a collecting foam is attached. The user independently inserts the foam into his/hers mouth for 60-90 seconds until it absorbs a sufficient amount of saliva, as indicated by the appearance of blue lines along the collecting foam. This collection procedure is simple and allows even young children to collect their own sample. Sample collection is now safer than ever, since no professional health-worker needs to approach individuals suspected to be infected. The in-mouth collection ensures a clean procedure, preventing biofluids spills in the sampling area. Furthermore, this collection system sets the ground for an easy, reliable and affordable home COVID-19 sample collection.

New Saliva Collection and Processing Device
the PCR machines. Thus, precious time and money are saved from the diagnostic procedure in the form of reagents, time of processing and workforce. In addition, reduced handling stages mean reduced number of mistakes, which results in a more accurate and consistent diagnostics system.
SAVING LOST SAMPLES
Bacterial proliferation is the major cause for mRNA instability and degradation, and for loss of PCR precious samples. It is currently met by refrigerating the samples and rapid transportation of them to the testing lab. In an additional set of experiments, it was shown that saliva samples that were subjected with Salignostics’ technologies had no bacterial growth at least for 72 hours at room temperature (opposed to non-treated saliva with widespread growths. See picture to the right). Elimination of bacterial growth opens new opportunities to logistically cope with collecting samples from broader population, in locations where refrigeration is not available. This will even further increase testing compliance as the procedure is not only more convenient, but also can be performed at a location of choice.
PRELIMINARY RESULTS AND FUTURE ASPECTS
In early experiments it was found that saliva that was collected and processed by Salignostics’ device yielded similar results to the ones yielded by saliva samples that underwent the Gold Standard procedure.
The Salignostics saliva collecting and processing device will potentially provide a powerful tool for both a better testing compliance and a better analytical procedure of COVID-19 tests. Thus, more people will be able and will be willing to be tested. The more individuals will be tested in more locations, the more accurate the epidemiological picture the healthcare officials will have. In turn, this will result with better decision-making system which will lead to an improved capability of controlling and managing the epidemic, for the sake of the general public.
Become an agent today, contact us using the form below!
Address:
ROOM 717, 7 FLOOR, TUNG LEE IND. BLDG. NO. 09 LAI YIP STREET, KWUN TONG, KOWLOON, HONG KONG
Email Us
Call Us
WHATSAPP: 852-5448-4997
